Medical PCBA Manufacturing: Reliable Solutions for Healthcare Electronics
In the fast-evolving world of healthcare technology, precision and reliability are not just preferences—they are critical requirements. Behind every life-saving device, from wearable monitors to advanced imaging systems, lies a complex network of components working seamlessly together. At the heart of this network is the medical PCBA, a small but essential element that ensures devices perform accurately and safely. As medical electronics become increasingly sophisticated, understanding how these assemblies are designed, manufactured, and maintained becomes vital for anyone involved in healthcare innovation.

Introduction to Medical PCBA
Definition and Importance
A Medical PCBA (Printed Circuit Board Assembly) is the combination of a printed circuit board (PCB) with all its electronic components soldered or mounted onto it, forming a functional unit that controls the electronic operations of a medical device. Unlike standard PCBAs, medical PCBAs are designed for high reliability, precision, and compliance with strict healthcare standards.
Medical PCBAs are critical in healthcare devices because they ensure that electronics operate accurately and consistently. Even minor failures in these assemblies can lead to incorrect readings, device malfunctions, or compromised patient safety. For example, imaging equipment like MRI or CT scanners relies on PCBAs to manage complex signal processing, while wearable medical devices such as heart rate monitors depend on them for continuous, precise measurements. Diagnostic instruments, including blood analyzers and glucose meters, also require reliable PCBAs to deliver accurate results in clinical settings.
Overview of Healthcare Electronics Market
The healthcare electronics market has grown rapidly due to rising demand for advanced medical devices and the global emphasis on patient-centered care. Analysts report that the market for high-reliability PCBAs in medical devices is expanding faster than in many other electronics sectors, driven by trends like wearable health monitors, telemedicine equipment, and AI-assisted diagnostics.
When comparing consumer electronics and medical electronics, the differences in requirements are stark. Consumer devices can often tolerate minor errors, occasional downtime, or less rigorous testing. In contrast, medical electronics demand near-perfect reliability, extensive testing, and regulatory compliance. For instance, a smartwatch that miscalculates a step count has limited consequences, but a cardiac monitoring device that fails due to a PCBA fault can endanger a patient’s life.
Role of PCBA in Patient Safety and Device Performance
The reliability of a medical PCBA directly impacts the safety and performance of healthcare devices. A well-designed PCBA ensures accurate data collection, proper signal processing, and consistent operation under various conditions.
Step by step, the effects of a faulty PCBA could include:
1. Data Errors – Sensors may produce incorrect readings, affecting diagnoses.
2. Device Malfunctions – Critical functions may fail, such as alarms in infusion pumps not triggering.
3. Treatment Risks – Incorrect dosage or intervention due to faulty signals could harm patients.
4. Regulatory and Legal Consequences – Device recalls and non-compliance can result from undetected PCBA defects.
By maintaining stringent quality control and using high-grade components, manufacturers ensure that medical PCBAs support patient safety, device accuracy, and overall healthcare effectiveness.
Key Requirements for Medical PCBA Manufacturing
Regulatory Standards and Compliance
Medical PCBAs must comply with strict regulatory standards to ensure safety, reliability, and legal approval. Key regulations include:
ISO 13485: This international standard defines requirements for a quality management system in medical device manufacturing. It ensures that PCBAs are consistently produced and controlled to meet patient safety standards.
IEC 60601: Focused on electrical safety and performance, this standard applies to medical electrical equipment, ensuring PCBAs do not pose electrical hazards.
FDA Regulations: In the United States, the FDA enforces compliance for medical device manufacturing, requiring documentation, validation, and traceability of PCBAs.
Examples of documentation required for regulatory approval include:
Design history files (DHF) detailing the PCBA design and iterations.
Risk management reports evaluating potential failures and mitigations.
Manufacturing process validation reports showing consistent production quality.
Traceability records linking each component to its batch or supplier.
Quality Assurance and Testing Protocols
Ensuring high-quality, reliable medical PCBAs requires comprehensive inspection and testing protocols. Common methods include:
Automated Optical Inspection (AOI): Uses cameras and software to detect defects such as misaligned components, solder bridges, or missing parts.
X-ray Inspection: Allows for non-destructive examination of solder joints, hidden components, and internal layers of the PCBA.
Stress testing is equally critical to guarantee long-term reliability:
Thermal Cycling: Simulates repeated heating and cooling to detect failures due to expansion and contraction of materials.
Vibration Tests: Ensures the PCBA can withstand physical shocks during transport or device operation.
Moisture Resistance Testing: Verifies that PCBAs perform correctly in high-humidity or sterilization environments.
These processes collectively ensure that each PCBA meets rigorous performance and safety requirements for medical devices.
Material Selection for Medical PCBA
Choosing the right materials is essential for durability, safety, and precision in medical PCBAs. Key materials include:
FR4: Standard high-quality fiberglass material used in many PCBs; cost-effective but may have limitations in extreme conditions.
Rogers: High-frequency laminates suitable for advanced signal processing in diagnostic equipment.
Polyimide: Flexible, heat-resistant material used for wearable or compact devices.
Other considerations include biocompatibility—materials must be safe for patient contact—and long-term stability, ensuring devices function reliably over their expected lifespan.
Comparison:
Standard PCB: Adequate for consumer electronics; tolerates minor deviations in performance.
Medical-grade PCB: Must meet strict safety, thermal, and chemical standards; supports life-critical applications and requires precise quality control.
Manufacturing Process of Medical PCBA
Design and Prototyping
The design and prototyping phase is the foundation of a reliable medical PCBA. One key concept is DFM (Design for Manufacturability), which ensures that PCB layouts, component placement, and circuit designs are optimized for efficient, error-free manufacturing. DFM also considers factors such as thermal management, signal integrity, and ease of assembly.
Example: When prototyping a wearable glucose monitor, engineers carefully select flexible PCB materials, place sensors to maximize accuracy, and design circuits to minimize power consumption. Prototyping allows testing of real-world performance before full-scale production, reducing the risk of costly errors.
Component Sourcing and Traceability
Sourcing certified, high-reliability components is critical in medical PCBA manufacturing. Using substandard or uncertified components can lead to device failure, regulatory non-compliance, or patient safety risks.
Step-by-step traceability process:
1. Supplier Qualification – Verify suppliers meet quality and regulatory standards.
2. Batch Documentation – Record serial numbers and batch codes for every component.
3. Assembly Tracking – Link each PCBA to the specific components used.
4. Audit Readiness – Maintain traceability logs for regulatory inspections or potential recalls.
Traceability ensures accountability and allows manufacturers to quickly identify and isolate defective components if needed.
Assembly Techniques
Medical PCBAs are assembled using precise techniques to ensure reliability and performance:
SMT (Surface Mount Technology): Ideal for high-density, small components commonly used in wearable or compact medical devices.
Through-Hole Assembly: Used for components requiring stronger mechanical support, such as connectors or large power modules.
Controlled environments are critical for contamination-sensitive devices. Many medical PCBAs are assembled in cleanrooms, where dust, moisture, and other contaminants are strictly controlled.
Example: Implantable devices like pacemakers require meticulous cleanroom assembly and rigorous handling protocols, while external monitoring devices such as pulse oximeters can tolerate less stringent conditions.
Testing and Validation
Before deployment, medical PCBAs undergo thorough testing and validation to ensure functionality and safety.
Key testing processes include:
Functional Testing: Verifies that all circuits and components perform as designed.
Burn-In Testing: Operates the PCBA under stress conditions to detect early-life failures.
End-of-Line Inspection: Final verification before shipping to ensure defect-free assemblies.
Use case: Micro-failures, such as intermittent solder connections or tiny component misalignments, can be detected during these tests, preventing device failures in critical medical applications.
Challenges in Medical PCBA Manufacturing
Miniaturization and Complexity
Modern medical devices often demand high-density interconnects and fine-pitch components, meaning PCBAs must fit more circuits into smaller spaces. This miniaturization improves device functionality and portability but introduces significant manufacturing challenges.
Comparison: Standard PCBs for consumer electronics can tolerate slightly larger component spacing and simpler layouts, making assembly easier and less prone to defects. In contrast, high-speed medical PCBAs require ultra-fine pitch placement, precise soldering, and advanced inspection methods to prevent short circuits or signal interference. High-density designs also complicate thermal management, which is critical in devices like implantable monitors or imaging sensors.
Reliability under Extreme Conditions
Medical PCBAs must operate reliably in extreme conditions, including high temperature, humidity, and electromagnetic interference (EMI) commonly found in hospital environments. Failure to withstand these conditions can compromise patient safety and device performance.
Step-by-step simulation process used by manufacturers:
1. Thermal Stress Testing – Expose PCBAs to repeated heating and cooling cycles to identify thermal expansion or contraction failures.
2. Humidity Exposure – Test in high-moisture environments to evaluate resistance to corrosion or short circuits.
3. EMI Testing – Apply electromagnetic interference to ensure the PCBA maintains signal integrity and accurate readings.
4. Mechanical Stress Simulation – Test vibrations and shocks to mimic handling, transport, and operational movements.
These tests help manufacturers ensure that medical PCBAs continue to function reliably under real-world hospital conditions.
Supply Chain Risks
The reliability of medical PCBAs also depends on a robust supply chain. Challenges include:
Component Shortages – Critical parts may be unavailable due to market fluctuations.
Counterfeit Parts – Unauthorized components can compromise safety and performance.
Quality Variation – Differences between suppliers can lead to inconsistent device behavior.
Mitigation strategies:
1. Multiple Sourcing – Use more than one certified supplier to reduce dependency on a single source.
2. Vendor Audits – Regularly evaluate supplier processes and certifications to ensure consistent quality.
3. Traceability Systems – Track all components from source to assembly, enabling quick recall or replacement if defects are detected.
By addressing these supply chain challenges proactively, manufacturers reduce risk and maintain high reliability for critical medical devices.
Innovations and Trends in Medical PCBA
Advanced Materials and Coatings
Medical PCBAs increasingly rely on advanced materials and protective coatings to enhance reliability and safety. Common solutions include:
Conformal Coating: A protective polymer layer applied over PCB components to shield against moisture, dust, and chemical exposure.
Gold Plating: Used on critical contacts to improve conductivity, reduce oxidation, and extend component life.
Biocompatible Materials: Essential for devices that come into direct contact with patients, ensuring safety and preventing allergic reactions.
Benefits: These materials and coatings improve long-term reliability, device safety, and regulatory compliance, particularly for high-stress applications such as implantable devices or intensive care monitoring equipment.
Smart and Connected Medical Devices
The rise of IoT-enabled and AI-driven medical devices is transforming PCBA design and functionality. Modern medical PCBAs are not just electronic circuits—they support:
Remote Monitoring: Devices can transmit patient data to healthcare providers in real time.
AI-Driven Diagnostics: Embedded algorithms analyze sensor data for early detection of health issues.
Interconnected Systems: Medical PCBAs can communicate with other devices and hospital networks for integrated care.
Example: Smart infusion pumps adjust drug delivery based on real-time patient vitals, while wearable heart monitors continuously track heart rhythms and alert clinicians to anomalies. These applications demand highly reliable, precise, and connected PCBAs.
Sustainable and Green Manufacturing
Environmental responsibility is becoming a key trend in medical PCBA manufacturing. Practices include:
RoHS Compliance: Restricts hazardous substances such as lead, mercury, and cadmium.
Lead-Free Solder: Reduces environmental impact while maintaining solder joint reliability.
Energy-Efficient Processes: Optimized manufacturing lines minimize energy consumption and waste.
Comparison: Traditional PCB assembly often relies on lead-based solder and less efficient energy practices, whereas eco-friendly methods improve sustainability without compromising device quality or reliability.
These trends reflect a broader push toward safer, smarter, and more sustainable medical electronics, meeting both regulatory standards and market expectations.
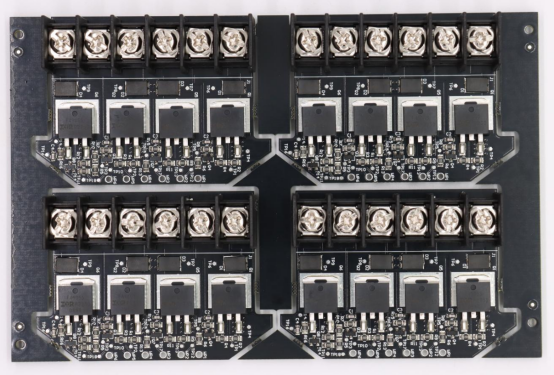
Choosing the Right Medical PCBA Manufacturer
Capabilities and Certifications
Selecting the right manufacturer starts with evaluating their capabilities and certifications. Reliable medical PCBA suppliers must comply with recognized standards, including:
ISO 13485: Ensures a quality management system specifically for medical devices.
UL Certification: Confirms electrical safety and product reliability.
CE Marking: Indicates compliance with European health, safety, and environmental standards.
Example checklist for evaluating a supplier:
1. Verified ISO 13485 certification and recent audit reports.
2. Evidence of UL or CE compliance for key products.
3. Capability to handle high-density, complex PCBAs.
4. Experience with regulatory documentation and quality assurance processes.
Manufacturers like PCBMASTER meet these standards, providing confidence that medical PCBAs will be produced safely and consistently.
Customer Support and Post-Manufacturing Services
Strong customer support and post-manufacturing services are essential for medical PCBAs due to their complexity and critical applications. Key services include:
Prototyping Support: Assistance in designing and producing initial PCBA prototypes.
Testing Assistance: Help with functional, environmental, and stress testing.
Repair/Rework Services: Quick resolution of defects or component issues after assembly.
Step-by-step evaluation of manufacturer responsiveness:
1. Contact the support team with a technical inquiry and measure response time.
2. Request examples of past prototyping or testing support.
3. Review rework and warranty policies for post-production issues.
4. Assess how quickly the manufacturer implements corrective actions.
Reliable support ensures issues are resolved promptly, minimizing delays and maintaining device safety.
Cost vs. Quality Considerations
Balancing budget constraints with high reliability is a crucial decision when choosing a medical PCBA manufacturer.
Comparison:
Low-Cost PCBAs: May compromise on material quality, testing rigor, or component traceability, increasing the risk of device failure.
Premium Medical-Grade PCBAs: Use certified components, advanced materials, and comprehensive testing, ensuring long-term reliability and regulatory compliance.
Investing in high-quality PCBAs reduces the likelihood of recalls, malfunctions, or safety incidents. PCBMASTER specializes in premium medical PCBAs, offering high-performance, reliable solutions while providing transparent cost structures.
Conclusion
Reliable medical PCBA plays a vital role in ensuring that healthcare electronics operate accurately, safely, and consistently. From wearable monitors to advanced imaging systems, the performance and safety of medical devices depend directly on the quality of their PCBAs. Achieving this requires strict compliance with regulatory standards, rigorous quality assurance practices, and the use of innovative materials and manufacturing techniques. While cost is a consideration, prioritizing reliability and compliance is essential to prevent device failures, recalls, or risks to patient safety. For anyone looking to learn more about PCBs and PCBAs, PCBMASTER provides professional guidance and solutions, supporting manufacturers in producing high-quality medical PCBAs that meet the highest industry standards.
FAQs
What makes medical PCBA different from standard PCB assembly?
Medical PCBA differs from standard PCB assembly in its higher reliability, stricter regulatory requirements, and enhanced safety standards. While consumer or industrial PCBs can tolerate minor defects or performance variations, medical PCBAs must operate accurately under critical conditions, often in life-supporting devices. They use high-quality materials, advanced assembly techniques, and extensive testing to ensure long-term performance. Examples include implantable devices, diagnostic instruments, and wearable health monitors, which require precision and durability far beyond typical electronics.
How do medical PCBAs ensure patient safety and device accuracy?
Medical PCBAs ensure patient safety and device accuracy through rigorous design, material selection, and testing protocols. High-quality components, precise soldering, and protective coatings prevent malfunctions. Functional and stress testing, including thermal cycling, vibration tests, and moisture resistance, verify that the PCBA performs reliably under real-world conditions. Accurate data collection and consistent operation of PCBAs in devices like infusion pumps or heart monitors directly contribute to correct diagnosis and treatment, reducing risks to patients.
What are the most common challenges in medical PCBA manufacturing?
Common challenges include:
Miniaturization and Complexity: High-density interconnects and fine-pitch components increase assembly difficulty and risk of defects.
Reliability under Extreme Conditions: PCBAs must withstand temperature fluctuations, humidity, and electromagnetic interference in hospital environments.
Supply Chain Risks: Component shortages, counterfeit parts, and quality variation can affect consistency and safety.
Addressing these challenges requires advanced design, careful material selection, and strict quality control throughout the manufacturing process.
How can manufacturers test and validate medical PCBAs effectively?
Manufacturers test and validate medical PCBAs using a combination of functional testing, burn-in testing, and end-of-line inspection. Step-by-step processes include:
1. Functional Testing: Confirm that all circuits and components operate as intended.
2. Stress Testing: Apply thermal cycling, vibration, and humidity exposure to simulate real-world conditions.
3. Burn-In Testing: Run devices for extended periods to detect early-life failures.
4. Final Inspection: Verify solder joints, component placement, and overall assembly quality before shipping.
This multi-layered approach ensures reliability, accuracy, and regulatory compliance.
Which certifications should a medical PCBA supplier have?
A reliable medical PCBA supplier should hold certifications including:
ISO 13485: Quality management system for medical device manufacturing.
UL Certification: Confirms electrical safety and product reliability.
CE Marking: Demonstrates compliance with European health, safety, and environmental standards.
These certifications ensure that the manufacturer meets regulatory requirements and can consistently produce high-quality, safe medical PCBAs.
